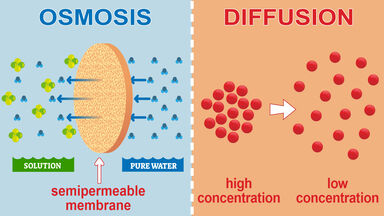
In Osmosis the particles of solvent molecules travel across the semipermeable membrane while in diffusion the molecules from a higher to lower concentration. The main difference is that diffusion does not involve particular structures while facilitated diffusion does.
Main Difference Between Osmosis And Diffusion In Biology
Tap again to see term.

. The roots of the plant absorbing water is a natural example of osmosis. Osmosis refers to movement across membrane diffusion doesnt need a membrane. Osmosis is dependent on one solvent to another for the reduction of free energy whereas in the case of diffusion the movement of molecules is from the area of their higher.
Diffusion and osmosis involves the movement of materials from one region another. Diffusion Osmosis aka cell transport Lab Objectives. It occurs only in liquid medium.
On one hand diffusion happens in any medium be it solid liquid or gas whereas osmosis can only happen in a liquid environment. Water molecules are the diffusing molecules in osmosis while diffusion can be solid gases and liquid. Diffusion movement of any substance osmosis only water.
One big difference between osmosis and diffusion is that both solvent and solute particles are free to move in diffusion but when we talk about osmosis only the solvent molecules water molecules cross the membrane. Active transport Osmosis diffusion. Osmosis is the diffusion of solvent mostly water through a semipermeable membrane down its concentration gradient.
From higher concentration to lower concentration. Click to see full answer. The process by which water molecules are able to diffuse through the cell membrane.
They each have different purposes as well. This movement could occur by. The main difference between osmosis and diffusion is that osmosis requires a semi permeable membrane.
Semipermeable membrane is required. Diffusion is the movement of molecules such as oxygen in and out of a cell. While diffusion can occur across areas that dont involve a semi-permeable membrane osmosis takes water in or out of a cell based on the concentration of surrounding water.
Diffusion is the process by which particles movie from high concentration gradient to lower concentration. According to these biological definitions osmosis is a form of diffusion. Diffusion is the movement of particles from a region of high concentration to low concentration while osmosis the movement of water molecules via a semi-permeable membrane from dilute to the concentrated solution.
Diffusion osmosis solute solvent solution kinetic theory of matter and selectively permeable. Click card to see definition. The example of diffusion is the spreading of perfume in the air when it is sprayed while in osmosis the best example is the spread of water in the roots of plants.
Moreover the goal of diffusion is to attain equilibrium in the energy concentration. It involves movement of solvent molecules only. Osmosis is a slow process and diffusion is a fast process.
Filtration is a process of transportation of substance passively between the compartments. It is influenced by turgor or hydrostatic pressure of the system. Osmosis is the movement of water molecules through the cell.
Tap card to see definition. The main difference between diffusion and active transport is that diffusion is a passive transport method in which molecules move across the cell membrane through a concentration gradient whereas active transport requires cellular energy in order to transport molecules against the concentration gradient. Movement of small molecules like water across cell membrane.
Diffusion Diffusion refers to the process in which particles from a higher concentration tend to move or transport to a lower concentration medium in order to attain the equilibrium. In osmosis the flow particles can occur only in one direction. 14 rows In biology osmosis is only discussed in terms of the movement of water through the cell membrane.
List the factors that influence permeability and the rate of diffusion Define hypotonic hypertonic isotonic and the effect they have on cells Analyze and explain the. The osmosis can take place only between similar types of solvents. Include in your answer which type of the molecule is moving for each process in which direction the molecules in question are moving low to high or high to low concentrations.
Aside from those said earlier one of the major differences between the two is the type of medium where these processes happen. Diffusion refers to the movement of molecules along concentration gradientie. Define andor explain the following concepts.
In diffusion the flow of particles can occur in all directions. Explain the major difference between diffusion and osmosis. Carbon dioxide oxygen water food substances wastes eg urea.
Osmosis regulates the water supply and hydration of animal and plant cells. Diffusion can occur between similar or dissimilar types of solvents. The movement of small molecules from a higher concentration to an area of lower concentration.
The swelling up of red blood cells when exposed to fresh water is another example of osmosis. The diffusion process can neither be stopped nor reversed. The main difference between the two is that diffusion can occur in any mixture even when two solutions arent separated by a semipermeable membrane whereas osmosis exclusively occurs across a.
14 rows The primary differentiating factor between the two systems is the medium in which they are. Substances move from a high to a low concentration down a concentration gradient. The movement of materials always occur in cells.
Though it is the diffusion of solvent molecules only yet influenced by the presence of other substances solutes in the system. Click again to see term.

What S The Difference Between Diffusion And Osmosis

What S The Difference Between Diffusion And Osmosis

Major Difference Between Osmosis And Diffusion Similarities Yb Study
0 Comments