Methane oxygen carbon monoxide nitrogen and helium all have different. Solubility is the ability of a solid liquid or gaseous chemical called the solute to dissolve in solvent usually a liquid and form a solution.
In turn polar solutes tend to dissolve best in polar solvents while non-polar solutes tend to dissolve best in.
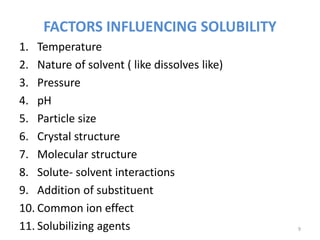
. FACTORS INFLUENCING SOLUBILITY 1. In general solubility of a gas in water will decrease with increasing temperature. Factors affecting drug stability.
Wei-Qin Tony Tong in Developing Solid Oral Dosage Forms 2009. Nature of solvent like dissolves like 3. Less the coefficient less is.
Enumerate the different factors affecting solubility - 1736818. In this video we learn about some of the factors that affect the solubility of solids and gases in waterThanks for watching. The 6 Factors Affecting Solubility Main.
Solvates hydrates 5salt form of drug 6. Acidic and alkaline pH influence the rate of decomposition of most drugs. 1Assuming that your setradioflat ironand refrigerator are connected in a series circuitwhat happened to the three remaining applian.
Environmental factors such as temperature humidity and light and to establish a retest period for the drug substance or a shelf life for the drug product and recommended storage conditions. In the stomach and intestine drug solubility can be enhanced by food and bile components such as bile salts lecithin and fatty acids. It is more difficult it is for solvent molecules to surround bigger molecules.
There are three main factors that control solubility of a solute. Several factors affect the solubility of gases. The larger the molecules of the solute are the larger is their molecular weight and their size.
It is critical to understand the most common factors that affect the stability of such analytes in order to properly develop methods for. Evaluation of the stability of drugs and drug metabolites in a biological matrix is a critical element to bioanalytical method validation. Factors that increase the amount of drug solubilized are particularly important for BDDCS class 2 and 4 drugs whose absorption is.
Addition of substituent 10. These are a few factors affecting the solubility of substances. Reading this you will be able to cover which factors affect solubility and how it affects the entire process of dissolution.
424 Dissolution of Salts in GI Fluids. Strong solute-solvent attractions equate to greater solubility while weak solute-solvent attractions equate to lesser solubility. Lipid water solubility coefficient is the ratio of dissolution of drug in lipid as compared to water.
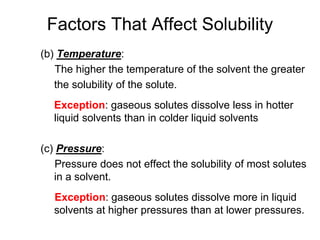
Drug solubility depends on the pH of fluid temperature volume and contents of fluid. High temperature accelerate oxidation reduction and hydrolysis reaction which lead to drug degradation 2. Temperature affects the solubility of both solids and gases but pressure only affects the solubility of gases.
You can increase or decrease factors like pressure temperature bonds and forces to increase or decrease the solubility rate of elements. The relation between the solute and solvent is very important in determining solubility. If all of the above mentioned factors ale excluded a general rule can be found that larger particles are generally less soluble.
Describe relationship between dielectric constant and solubility. Solute-Solvent Interactions Affect Solubility. B Partition coefficient d Solubility of electrolytes 12.
Polymorphism amorphism 4. Many drugs are stable between pH 4 and 8. Please like and subscribe.
Colder water will be able to have more gas dissolved in it. There are two direct factors that affect solubility. Solubilities of Gases in Water.
The lipophilicity of a drug is also correlated with water solubility. Factors that may affect the extent of solubilization. Solute- solvent interactions 9.
Although the pharmaceutical scientist plays a critical role in determining the stability of pharmaceuticals practicing pharmacists should be able to. Factors That Affect Solubility Ex5 As the temperature of a liquid increases the solubility of most solids in the liquid will 1 increase 2 decrease 3 remain the same Ex6 The process by which ionic solutes are surrounded by water molecules is called 1 polymerization 3 distillation 2 hydration 4 ionization Ex7 The reason why many salts dissolve in water is that. The rate and extent of absorption can be altered by food.
Up to 24 cash back FACTORS AFFECTING DRUG ABSORPTION List of content -- IPHARMACEUTICAL FACTORS A Chemical factors B Physicochemical properties of drug substances 1drug solubility dissolution rate 2. Explain factors affecting solubility of gases in liquids. One of these factors is temperature.
The size of the solid particle influences the solubility because as a particle becomes smaller the surface area to volume ratio increases. Describe preparation of saturated solution for determination of solubility. The factors affecting absorption of drugs are related both to the drugs and to the body.
Depending on their properties the degree of solubilization for different drugs varies. Main Factors affecting solubility Are the polarity the effect of the common ion the temperature the pressure the nature of the solute and the mechanical factors. Greater the lipid water solubility coefficient more is the lipid solubility of the drug and greater is the absorption.
1 Temperature 2 Nature of solute or solvent 3 Pressure. Surface area does not affect how much of a solute will be dissolved but it is a factor in how quickly or slowly the substance will dissolve. Factors affecting solubility-The solubility depends on the physical form of the solid the nature and composition of solvent medium as well as temperature and pressure of system6.
Factors Related to Drugs. Particles size effective surface area 3. Explain the temperature dependence of gas solubility in liquid solutions.


0 Comments